


Subhra Pharma Pvt. Ltd. is a well established organisation dealing in the wide range of activities related to pharmaceutical industry.
We are totally committed to set our own standards in our work, which are among the best in industry. We believe that the man behind the machine makes a product great. Our strength lies in our team of people and its intellectual resources.
At Subhrapharma, our motto is to improve the health and well-being of people around the world by exporting innovative, high-quality pharmaceutical products that meet the needs of patients and healthcare professionals. We are committed to being a trusted partner to healthcare providers, regulatory agencies, and other stakeholders, and to operating ethically, with a commitment to social responsibility and environmental sustainability.
Subhrapharma has a strong track record of exporting innovative products that address unmet medical needs. Our product portfolio includes a range of prescription drugs, over-the-counter medications, and medical devices, which are exported to countries around the world.
We are dedicated to delivering exceptional value to our customers and partners, and to building long-term relationships based on trust and mutual respect. Thank you for choosing Subhrapharma. We look forward to working with you to improve the health and well-being of people around the world.

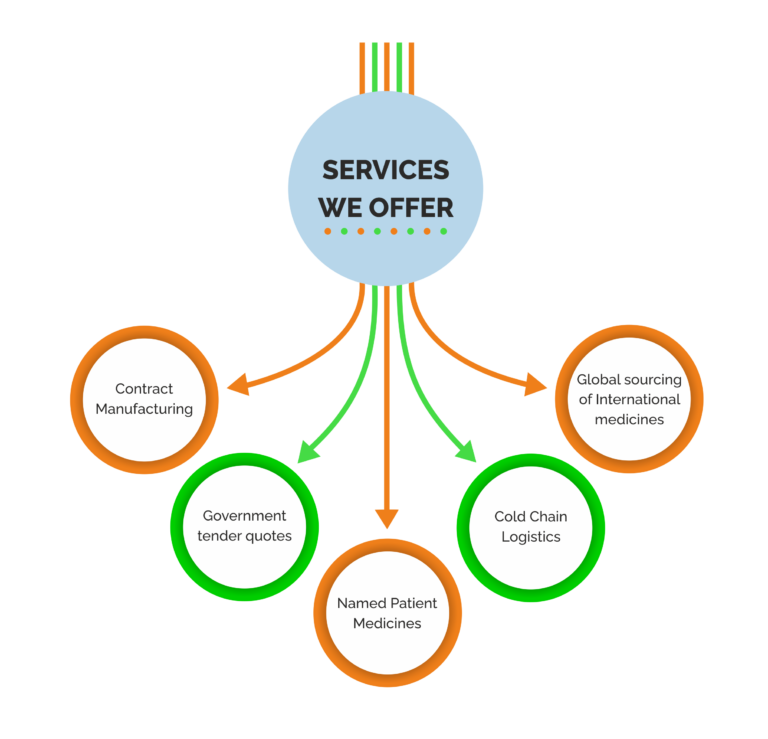





Exporting goods from India can be a lucrative and exciting opportunity for businesses around the world. India is known for its diverse range of products and has a strong manufacturing base, making it an attractive source of goods for international markets.
Cold chain logistics is a crucial element of the supply chain for the pharmaceutical industry, as it helps to ensure that temperature-sensitive medicines are of the highest quality and safety. Cold chain logistics helps to ensure that these medications are stored and transported in a way that preserves their quality and effectiveness.

Named patient medicines, also known as compassionate use medicines or expanded access medicines, are medications that are made available to patients outside of clinical trials for the treatment of a serious or life-threatening condition for which no satisfactory alternative treatment is available. These medications are typically not yet approved for general use by regulatory agencies such as the US Food and Drug Administration (FDA) or the European Medicines Agency (EMA).





